Health Ministry recalls vancomycin, phenylephrine, fentanyl IV bags
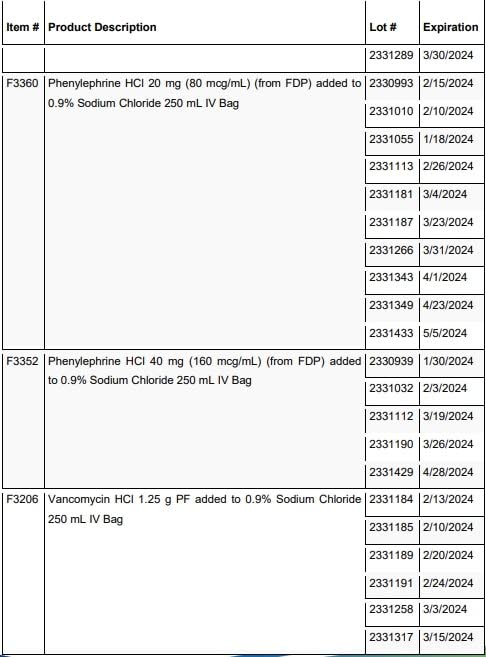
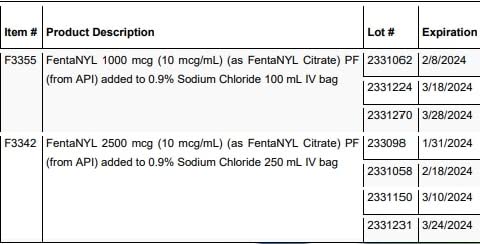
The Health Ministry has issued a voluntary recall of 33 lots of vancomycin, phenylephrine and fentanyl intravenous bags through its Chemistry, Drug and Food Division, as they may contain twice the recommended dosage of the drugs.
In a release on Tuesday, the ministry said high doses can have adverse effects, including serious adverse cardiac events, respiratory depression, and even death.
It said the products were distributed within the US to hospitals for administration in hospitals and are not registered for use in Trinidad and Tobago. It said no permits were issued for the importation of vancomycin and fentanyl.
“Out of an abundance of caution, the ministry advises persons who may be in possession of the recalled product to discontinue use immediately and to return the product to the place of purchase where possible,” it said.The ministry said it would continue to monitor the situation and advise the population as necessary.
A full list of the drugs being recalled is on the Ministry of Health’s website or social media pages.
Additional information can also be obtained by contacting the Office of the Director of the Chemistry, Food and Drugs Division at cfdd@health.gov.tt or 217-4664 ext. 13101 or the Drug Inspectorate at drug.inspectoratett@gmail.com or 217-4664 ext 13401.

Comments
"Health Ministry recalls vancomycin, phenylephrine, fentanyl IV bags"