[UPDATED] Health Ministry issues guidelines on covid19 rapid test kits
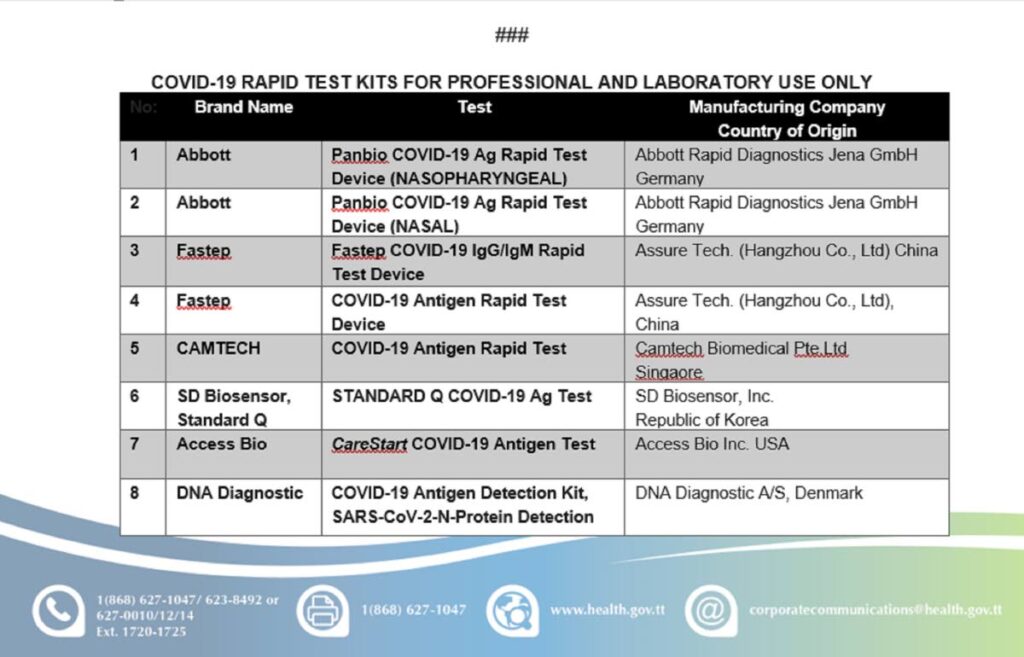
The Health Ministry has issued a list of the rapid antigen/antibody test kits that have received a no-objection letter from the Chemistry, Food and Drugs Division (CFDD) for importation into TT. The test kits listed are meant for professional and laboratory use only.
In a release, the ministry reminded the population that before importing any rapid antigen or antibody test kits, an application together with supporting documents and a sample of the product must be submitted to the division for an assessment and a “no objection” letter issued.
It said under the Food and Drugs Act, Chapter 30:01, “the term “device” refers to any instrument, apparatus or contrivance, including components, parts and accessories thereof, manufactured, sold or represented for use in the diagnosis, treatment, mitigation or prevention of a disease, disorder, abnormal physical state, or the symptoms thereof, in man or animal. Rapid antigen/ antibody test kits fall within this definition.”
The approved rapid tests are under the brand names Abbott, Fastep, CAMTECH, SD Biosensor - Standard Q (Korea), Access Bio, DNA Diagnostic, VivaChek, QUIDEL, ARIA, SURE STATUS, Co-Diagnostic Inc, EUROIMMUN, SD Biosensor (Mannheim), BD Veritor TM System, and SD Biosensor (Korea).
It said the process for issuing a no-objection letter to import devices was: once an application for a letter with relevant supporting documents was submitted to CFDD, the division will process the application to determine compliance with the Food and Drugs Act, and the applicant will be notified as to whether the application has been accepted (and the letter is issued) or rejected.
It said a no-objection status can be amended on the basis of new data and information arising about the safety or quality of the test kit. Where a rapid antigen/antibody test kit is imported without the relevant approvals by the CFDD, the importer may be liable to a fine and/ or imprisonment under the Food and Drugs Act.
It recommended that the PCR test remain the internationally accepted confirmatory test for covid19 detection and diagnosis.
It also cautioned importers, healthcare providers and the public about the limitations of rapid antigen/antibody test kits as the sole basis for diagnosing patients with the covid19 virus.
Further information on the application process can be obtained from the CFDD at cfdd@health.gov.tt or 868-623-5242.
The public is encouraged immediately to report cases of misleading or deceptive labels and advertisements which may create an erroneous impression of the character, value, composition, merit or safety of a test kit to the CFDD at cfdd@health.gov.tt or 868-623-5242.


Comments
"[UPDATED] Health Ministry issues guidelines on covid19 rapid test kits"